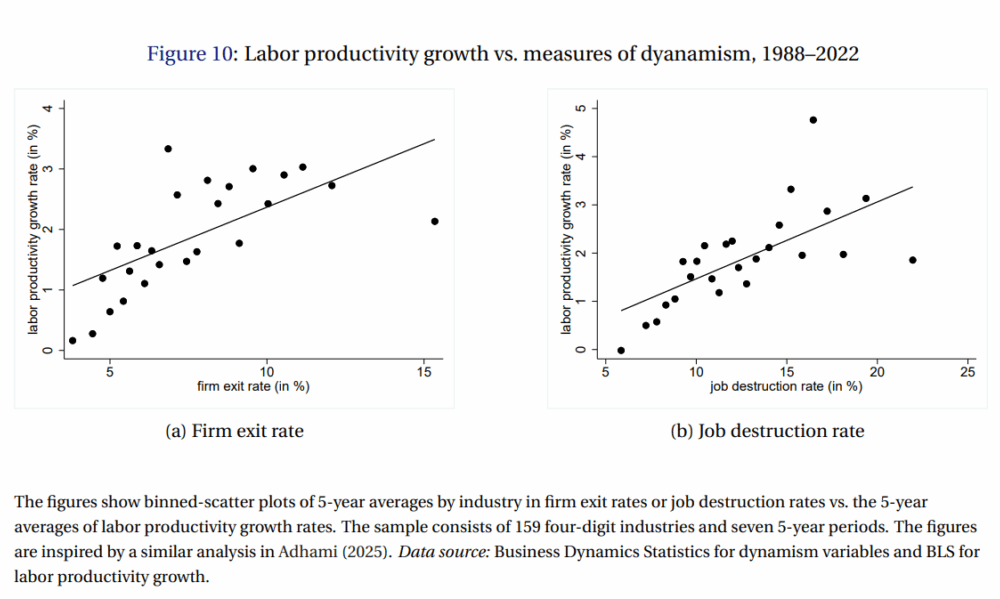
A recent study has provided valuable insights into the real-world commercial implementation of gene therapies for both sickle cell disease and beta thalassemia. Presented at the 67th ASH Annual Meeting and Exposition held from December 6 to 9, 2023, in Orlando, Florida, the research outlines critical lessons learned that can inform best practices as manufacturers and medical centers gear up to meet the increasing demand for these innovative treatments.
As the healthcare landscape evolves, the significance of gene therapies in treating genetic disorders has become increasingly apparent. This study underscores the importance of understanding the practical implications of implementing such therapies in clinical settings. It aims to assist stakeholders—including pharmaceutical companies and healthcare providers—in preparing for a future where gene therapies could transform treatment paradigms for patients with sickle cell disease and beta thalassemia.
Key Findings from the Study
The findings highlight several challenges and opportunities in the rollout of gene therapies. Notably, the study emphasizes the necessity of establishing robust supply chains to ensure that therapies are accessible to patients in need. It also points out the importance of training healthcare professionals to manage these complex treatments effectively.
Additionally, the research provides data on patient outcomes, showcasing the potential for gene therapies to significantly improve quality of life for individuals affected by these conditions. With the global prevalence of sickle cell disease and beta thalassemia, the need for effective treatment solutions has never been more urgent.
Experts involved in the study advocate for increased collaboration between manufacturers and healthcare providers. By sharing best practices and lessons learned, stakeholders can create a more cohesive framework that facilitates the successful integration of gene therapies into existing treatment protocols.
Looking Ahead
As demand for gene therapies grows, the insights from this study are expected to play a crucial role in shaping future strategies. The researchers call for ongoing evaluation of the commercial rollout process, encouraging continuous feedback from both patients and providers to refine treatment approaches.
The implications of this research extend beyond immediate patient care. They signal a critical shift in how genetic disorders are treated, paving the way for more personalized and effective healthcare solutions. As the field of gene therapy continues to evolve, the lessons learned from this study will be instrumental in addressing the challenges that lie ahead.