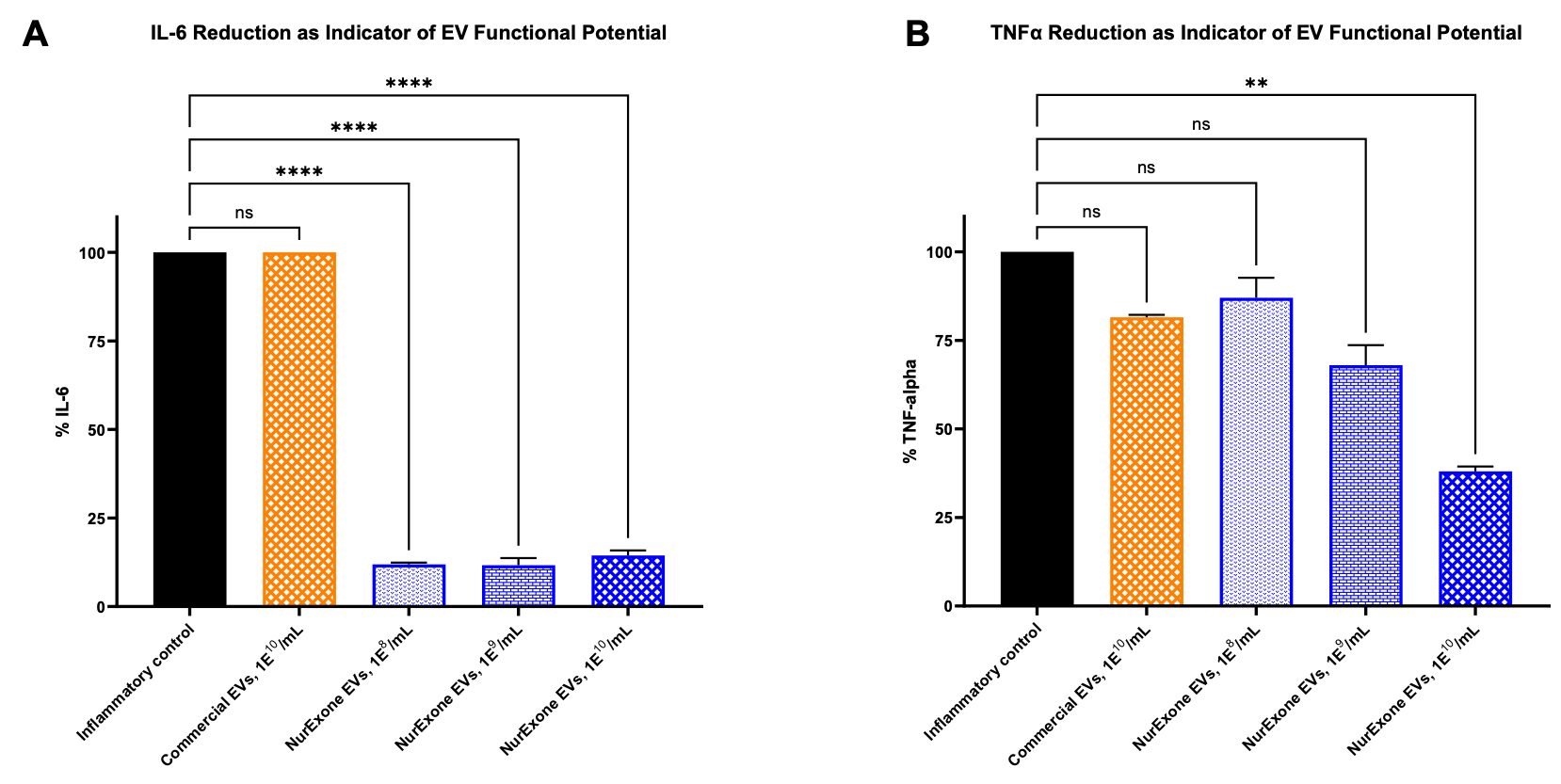NurExone Biologic Inc. has reported significant findings from recent laboratory analyses that demonstrate the ability of its proprietary exosomes to reduce inflammatory activity. According to the company, these exosomes show greater efficacy in suppressing inflammation compared to both untreated cells and a commercially available exosome product.
Dr. Tali Kizhner, the Director of Research & Development at NurExone, emphasized the importance of these findings, stating, “Inflammation and regeneration are conflicting biological processes, and this is particularly relevant in the Central Nervous System.” The laboratory results reveal that NurExone’s exosomes consistently outperform untreated cells and commercial alternatives, particularly as concentrations increase.
Significant Laboratory Findings
These results build on previously shared data from December 12, 2025. That analysis highlighted that exosomes derived from human bone marrow–derived mesenchymal stem cells exhibited superior biological activity compared to a commercial counterpart. Dr. Lior Shaltiel, Chief Executive Officer of NurExone, noted the company’s commitment to developing a robust analytical framework. This framework aims to quantify the biological complexity of exosome-based therapeutics, ensuring consistency and quality across production batches.
In the laboratory analysis, immune cells were stimulated to create a strong inflammatory response. This controlled environment allowed for a comparative evaluation of inflammatory signaling after treatment with both NurExone-produced exosomes and the commercially available product. Statistical analysis was conducted using one-way analysis of variance (ANOVA) to assess the significance of the results.
The findings indicated that NurExone’s exosomes reduced levels of the inflammatory signaling molecule IL-6 by more than 86% compared to untreated inflamed cells, even at the lowest concentration tested. This reduction was consistent across all tested concentrations, underscoring the strong intrinsic anti-inflammatory activity of the exosomes. For another inflammatory marker, TNF-alpha, the exosomes exhibited a clear concentration-dependent response, achieving reductions of over 60% at the highest concentration evaluated.
In contrast, the commercially available exosome product showed negligible reductions in both IL-6 and TNF-alpha levels, demonstrating the superior effectiveness of NurExone’s exosomes.
Future Implications and Company Overview
NurExone’s advancements in exosome technology are positioned to play a crucial role in regenerative medicine, particularly for Central Nervous System injuries. The company’s lead product, ExoPTEN, has shown strong preclinical data, supporting its potential for treating acute spinal cord and optic nerve injuries—both substantial markets estimated in the billions of dollars.
The company has achieved regulatory milestones, including obtaining Orphan Drug Designation, facilitating the pathway toward clinical trials in both the United States and Europe. NurExone is also focusing on establishing Exo-Top Inc., a U.S. subsidiary, to bolster its North American operations and growth strategy.
Dr. Lior Shaltiel remarked on the importance of these developments, stating, “This analytical framework will not only advance our drug programs, such as ExoPTEN but will also establish a reliable, scalable platform for exosome-based drug delivery.”
As NurExone continues to build on these promising findings, the future of its exosome-based therapies appears to be filled with potential for innovation in treating complex medical conditions. For further information about NurExone, interested parties may visit the company’s website or follow them on various social media platforms.
For media inquiries, contact:
Dr. Lior Shaltiel, Chief Executive Officer
Phone: +972-52-4803034
Email: [email protected]
Dr. Eva Reuter, Investor Relations – Germany
Phone: +49-69-1532-5857
Email: [email protected]
Allele Capital Partners, Investor Relations – U.S.
Phone: +1 978-857-5075
Email: [email protected]
NurExone’s recent studies contribute to a growing body of evidence supporting the therapeutic potential of exosomes in regenerative medicine, paving the way for future clinical applications.