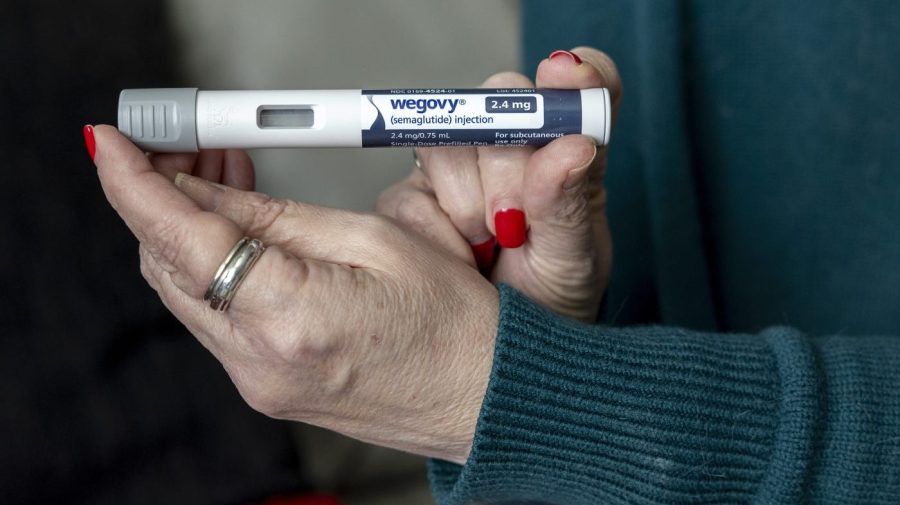
The Food and Drug Administration (FDA) has granted approval to Novo Nordisk for its oral version of the Wegovy weight-loss medication. This marks a significant milestone as it is the first oral supplement of its kind approved for public use. The announcement was made on March 5, 2024, and highlights a new option for individuals seeking to manage their weight effectively.
Wegovy, originally available as a once-weekly injection, contains the active ingredient semaglutide, which mimics the effects of the hormone GLP-1. This hormone is known to regulate appetite and food intake. The newly approved oral formulation is designed for once-daily consumption, allowing for greater convenience for users. According to Novo Nordisk, the oral GLP-1 pill is intended to “reduce excess body weight and maintain weight reduction long-term, while also reducing the risk of weight-related comorbidities.”
The Impact of Wegovy’s Oral Formulation
The approval of the oral Wegovy pill represents a significant advancement in weight management solutions. In clinical trials, participants using the injectable version of Wegovy experienced an average weight loss of around 15% of their initial body weight over 68 weeks. The oral formulation is expected to provide similar benefits, making it a promising alternative for those who may be hesitant to use injections.
Novo Nordisk’s release mentions that the oral version will be marketed as part of a comprehensive weight management program, which includes lifestyle changes such as diet and exercise. This approach aligns with current health guidelines that emphasize the importance of a holistic strategy for effective weight management.
The company anticipates that this new formulation will expand access to Wegovy, particularly for those unable or unwilling to use injections. As obesity rates continue to rise globally, the introduction of an oral option could prove crucial in addressing this public health issue.
Looking Ahead: Availability and Pricing
Although specific pricing details have yet to be disclosed, Novo Nordisk has indicated that the oral Wegovy will be available in pharmacies later this year. The company aims to ensure that the medication is accessible to individuals who could benefit from it.
The FDA’s approval reflects a growing recognition of the need for effective weight management solutions in today’s society. With obesity linked to various health complications, including diabetes and heart disease, the introduction of the oral Wegovy pill could play a vital role in improving health outcomes for many individuals.
As Novo Nordisk prepares for the launch, healthcare professionals and patients alike are eager to see how this new formulation will be received. With its potential to change the landscape of weight loss treatments, the oral Wegovy pill may become a key player in the ongoing fight against obesity.