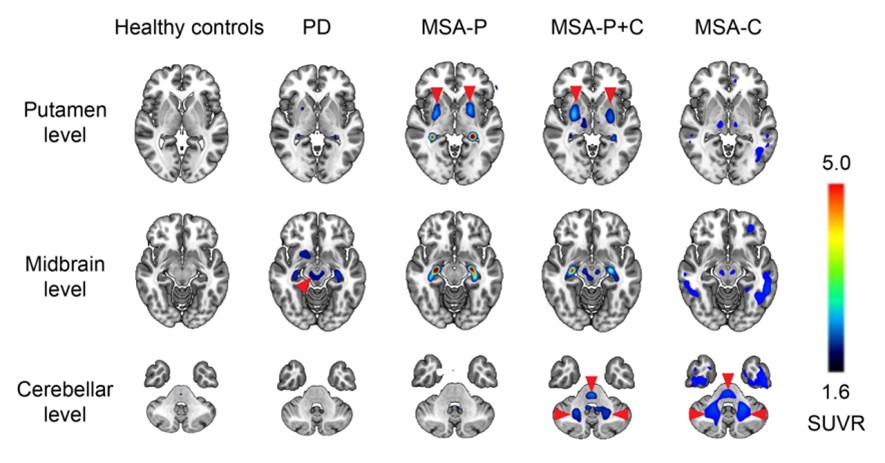
SynuSight Biotech, ABLi Therapeutics, and XingImaging have announced a strategic collaboration to incorporate alpha-synuclein PET imaging into clinical trials assessing the efficacy of risvodetinib as a treatment for Parkinson’s disease. This partnership, formalized on January 5, 2026, will utilize the proprietary PET tracer 18 F-FD4 to evaluate the clearance of alpha-synuclein from the brain, a key pathological marker in the disease.
The agreement allows ABLi to employ 18 F-FD4 in its clinical trials, which are focused on the potential of risvodetinib as a disease-modifying therapy. SynuSight has developed this PET tracer to visualize alpha-synuclein aggregates, providing crucial insights into the disease’s progression. As part of this arrangement, SynuSight will receive an upfront payment along with ongoing licensing fees from ABLi.
XingImaging will serve as the U.S. manufacturer for 18 F-FD4, integrating it with its state-of-the-art NeuroEXPLORER imaging technology at its facility in New Haven, Connecticut. This collaboration aims to create a comprehensive approach to assess the therapeutic effects of risvodetinib by combining molecular imaging with innovative blood-borne and tissue biomarkers developed by ABLi.
Clinical Trials Set for 2026
ABLi plans to implement 18 F-FD4 in two pivotal clinical trials in 2026. The first, named ABILITY-PD, aims to re-enroll 120 participants from a previous Phase 2a trial, which concluded in the fourth quarter of 2024. This study will focus on a longitudinal analysis of biomarkers related to brain, blood, and tissue over a 12-month period. It will also gather functional data related to motor and non-motor symptoms to correlate these with changes in biomarkers during treatment.
The second trial, known as the c-Abl inhibitor Modification of Parkinson’s Disease (CAMPD) trial, is a Phase 2b/3 study that will evaluate risvodetinib in a double-blind, placebo-controlled setup involving up to 500 participants with untreated Parkinson’s disease.
“This collaboration represents a key milestone in the global development of 18 F-FD4, integrating molecular imaging technologies with rigorously designed clinical trials,” stated Roger Fan, CEO of SynuSight. He emphasized the partnership’s goal to explore the disease-modifying potential of risvodetinib at a pathological level.
Potential Impact of Risvodetinib
Risvodetinib (also referred to as ABLi-148009) is a selective small-molecule inhibitor targeting non-receptor c-Abl kinases, aimed at addressing the biological mechanisms behind Parkinson’s disease. It is designed for once-daily oral administration and is thought to not only halt disease progression but also reverse functional loss associated with the condition.
Currently, treatment options for Parkinson’s primarily focus on managing symptoms, with no existing therapies proven to slow or halt the disease’s progression. Risvodetinib stands out as the first monotherapy to demonstrate improved patient quality of life in a randomized, placebo-controlled trial (NCT05424276) while also reducing underlying synuclein aggregate pathology in untreated patients. The compound is protected by intellectual property rights extending beyond 2036.
The PET tracer 18 F-FD4 has shown promise in preclinical studies, demonstrating effective blood-brain barrier penetration and producing high-contrast signals in relevant brain regions. In 2025, it received a significant boost with a $3.84 million grant from the Michael J. Fox Foundation for Parkinson’s Research, underscoring its potential as a next-generation imaging biomarker. Clinical development for this tracer is anticipated to commence in both the U.S. and China in early 2026.
This collaboration among SynuSight, ABLi, and XingImaging highlights the continued efforts to enhance understanding and treatment options for Parkinson’s disease, which affects millions worldwide. By combining advanced imaging techniques with innovative therapeutics, these organizations aim to unlock new pathways for effective disease management.